Top Notch Info About What Is A Standard Curve Graph Used For How To Make X Axis Words In Excel
:max_bytes(150000):strip_icc()/LognormalandNormalDistribution1-7ffee664ca9444a4b2c85c2eac982a0d.png)
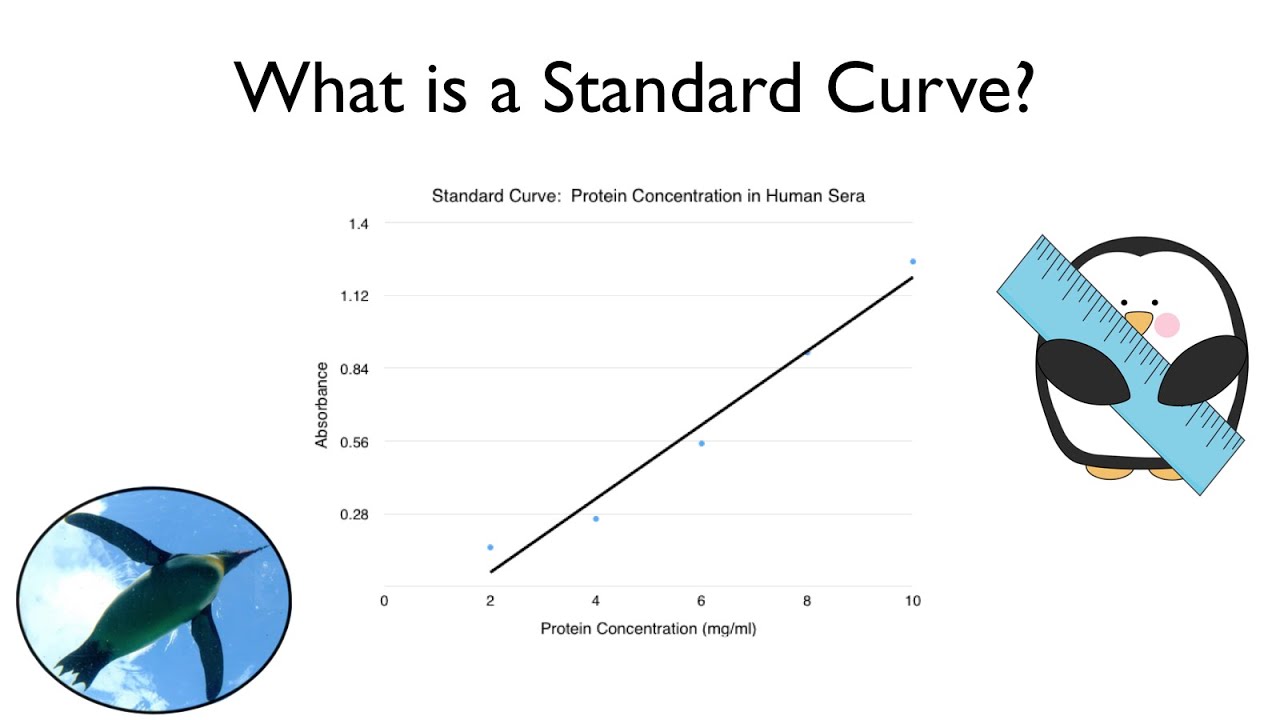
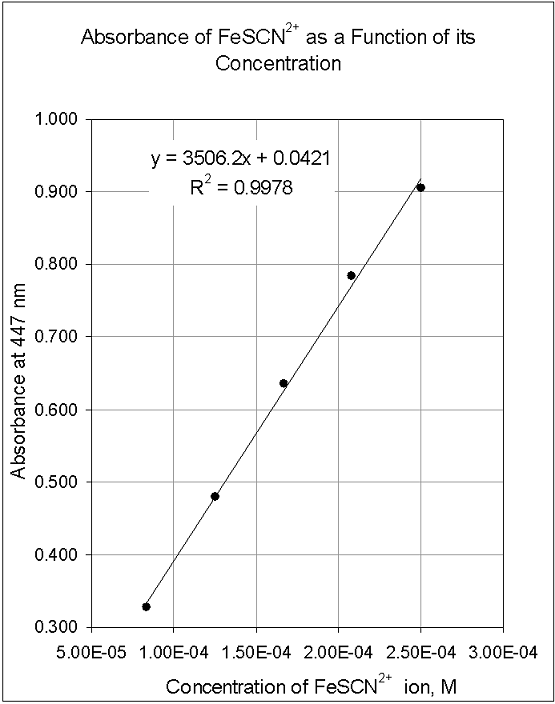
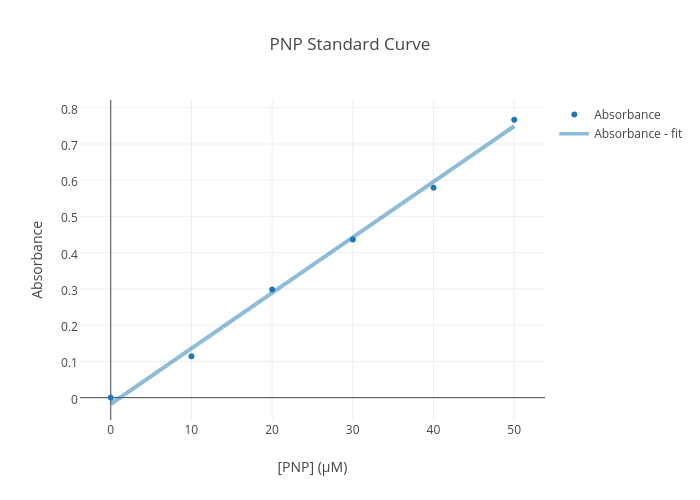
These curves use data points of known substances at varying concentrations, and researchers or developers can use these curves to find where an unknown substance plots.
What is a standard curve graph used for. Click the card to flip 👆. The standard curve slope is directly related to assay accuracy and sensitivity. Calculating unknown concentrations using a standard curve in prism 3.
A technical guide to standards, calibration curves and dilutions for the laboratory user. Oratory setting often refers to an actual chemical or physical material called a metro. In analytical chemistry, a calibration curve, also known as a standard curve, is a general method for determining the concentration of a substance in an unknown sample by comparing the unknown to a set of standard samples of known concentration.
A calibration curve is a graph used in analytical chemistry to determine the concentration of unknown substances in a sample by comparing them to a set of standard samples with known concentrations. For most protein assays, the standard curve is steepest (i.e., has the greatest positive slope) in. All else being equal, the steepest part of the curve is the most reliable.
Standard curve a standard curve is a quantitative research tool, a method of plotting assay data that is used to determine the concentration of a substance, A standard curve is a graph that shows the relationship between the concentration of a substance and its corresponding response, which can be measured quantitatively using absorbance, fluorescence, or luminescence methods. Multivariate calibration curves are prepared using standards that contain known amounts of both the analyte and the interferent, and modeled using multivariate regression.
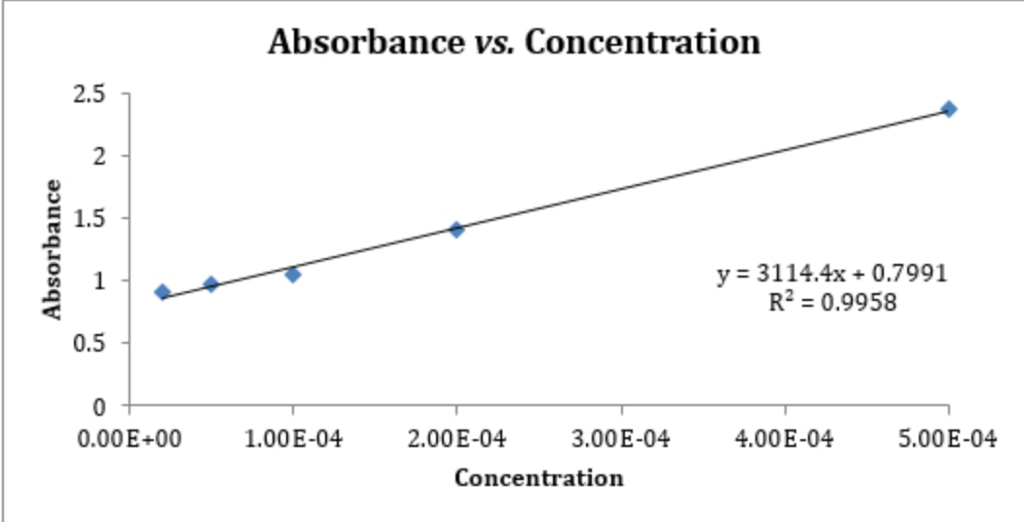
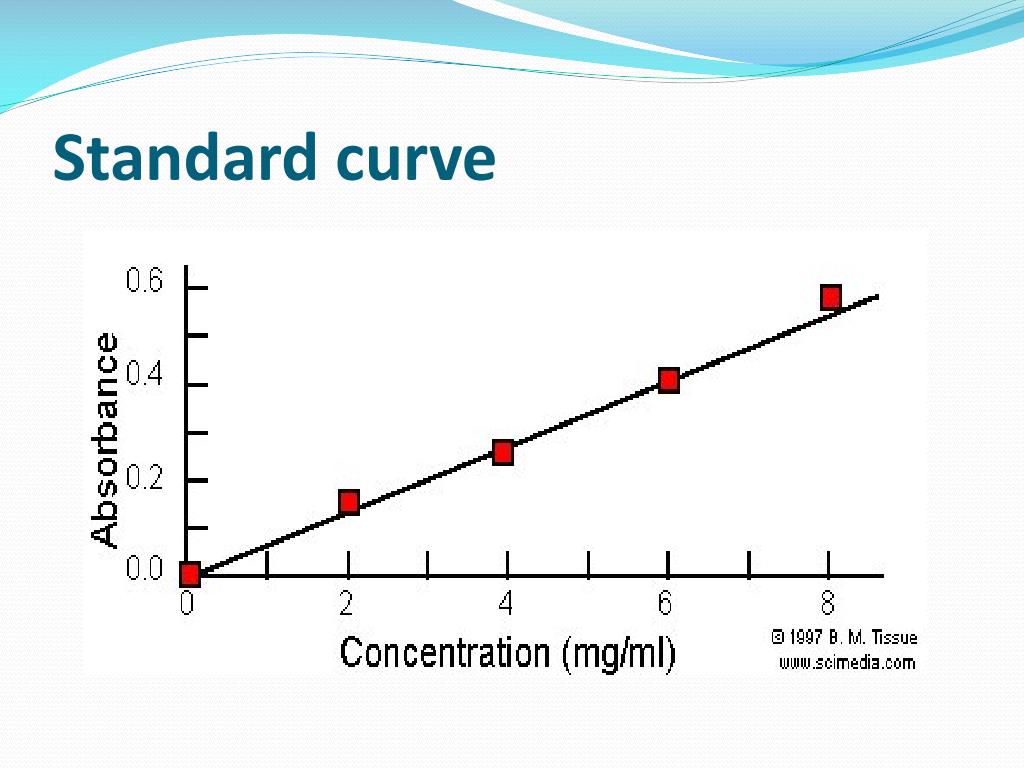
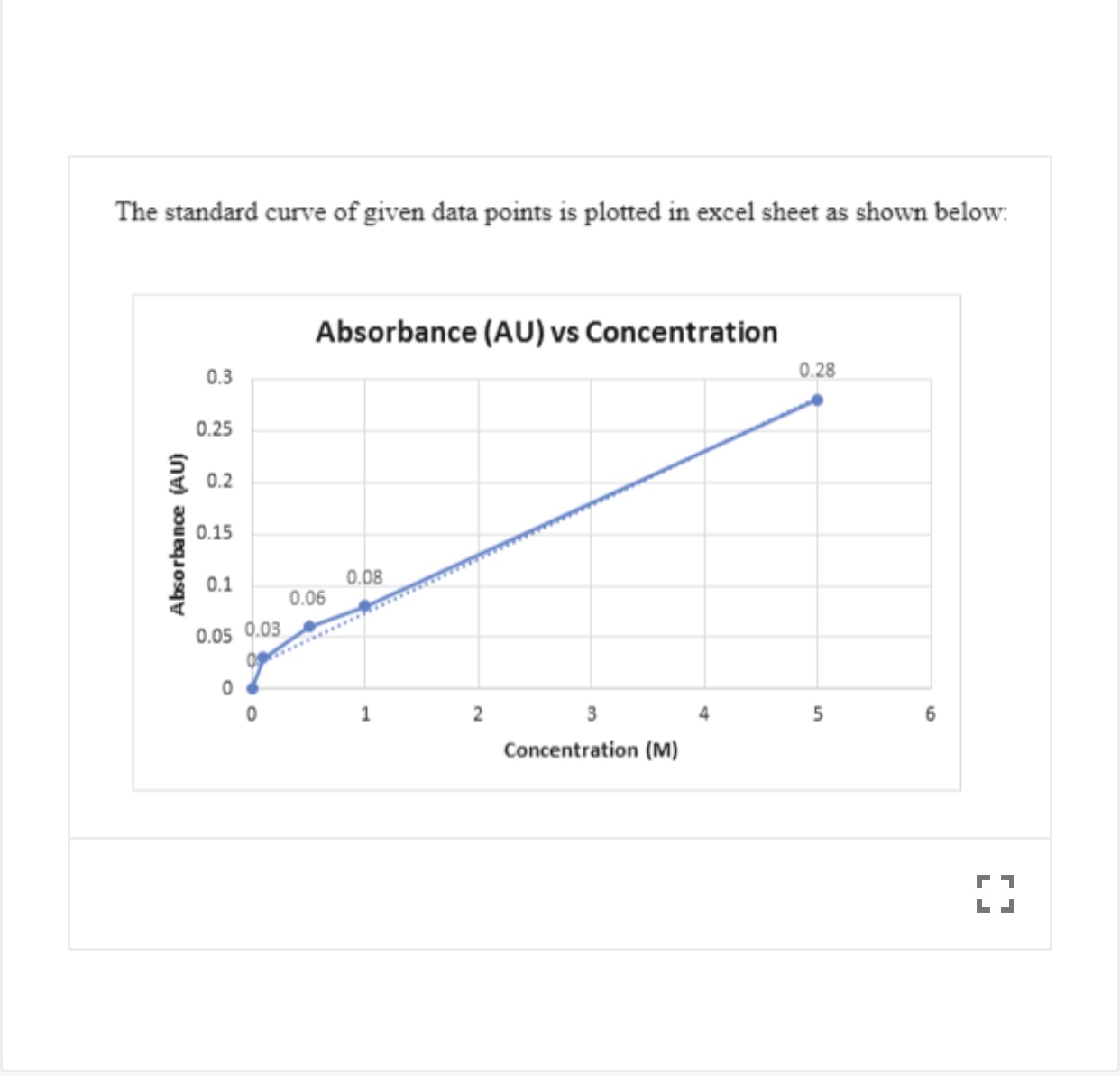
The standard curve will be used in part 3 of the lab to determine the concentrations of unknown solutions of methylene blue. Standard curves (sometimes called calibration curves) are incredibly important in research and medical diagnostics for quantifying an analyte. This guide will describe the process for preparing a calibration curve, also known as a standard curve.
A plot of any appropriate parameter against known amounts (or concentrations) of detectable substance. Use a standard curve to determine the values for unknown solutions. On a lcms or hplc each daily run should have a standard cur.
Why does a standard curve need to be run so often? A metrological standard is the fundamental example or reference for a. Calibration curves are used to understand the instrumental response to an analyte, and to predict the concentration of analyte in a sample.
In the case of the bradford protein assay, it will give the relationship between the amount of protein and the absorbance values. A standard curve is used to accurately determine the concentration of your sample from the signal generated by an assay. Identically assayed samples are directly comparable (“equal treatment means equal opportunity.”) sample assay responses are directly comparable to each other if they are processed in exactly the same manner.
Every standard curve is generated using a blank. Finding the concentration of an unknown by comparing it to a graph made from known concentrations of the same thing. But at a minimum you.
The importance of a standard curve. A standard curve is designed to correct for these effects, so you know which concentration a given signal value corresponds to. A calibration curve is created by first preparing a set of standard solutions with known concentrations of the analyte.